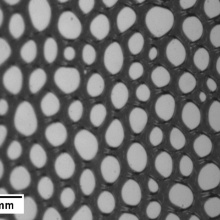
In order to gain a deeper knowledge on liquid foams, we study foams stabilized with pure and technical surfactants, and surfactant mixtures, respectively [1-11]. The influence of the surfactant structure, the surfactant and salt concentration as well as of the pH-value [4] on the foam properties are of particular interest. Our last studies show that hydrogen bonds between neighbouring surfactant molecules significantly enhance foam stability [1-4]. Therefore, we carry out the systematic foaming study for different surfactants. One part of them are capable of forming H-bonds and the other one not. Our focus is on foam properties such as (a) foamability (generating power of a surfactant solution), (b) foam stability (decay of foam as a function of time), and (c) foam drainage (change of liquid fraction in the foam because of gravity). For this purpose, we use the commercially available FoamScan [6] that applies image analysis and conductivity measurements to monitor the foam properties. In addition, the bubble sizes and bubble size distributions can be analysed with the new Cell Size Analysis (CSA) function [6], which allows a visualisation of the destabilizing processes (coarsening, coalescence and drainage).
| [1] How promoting and breaking intersurfactant H-bonds impacts foam stability, N. Preisig, T. Schad, L. Jacomine, R. Bordes, C. Stubenrauch, Langmuir, 2019, 35(47), 14999‑15008. |
|
[2] On the influence of intersurfactant H-bonds on foam stability: A study with technical grade surfactants, D. Ranieri, N. Preisig, C. Stubenrauch, Tenside Surf. Det., 2018, 55, 6-16. |
|
[3] On How Hydrogen Bonds Affect Foam Stability, C. Stubenrauch, M. Hamann, N. Preisig, V.Chauhan, R. Bordes ACIS, 2017, 247, 435-443. |
|
[4] Effect of protonation on foaming properties of dodecyldimethylamine oxide solutions: a pH-Study, K. Schellmann, N. Preisig, P. Claesson, C. Stubenrauch, Soft Matter, 2015, 11, 561-571. |
|
[5] Free Drainage of Aqueous Foams stabilized by Mixtures of a non-ionic (C12DMPO) and an ionic (C12TAB) surfactant, E. Carey, C. Stubenrauch, COLSUA, 2013, 419, 7-14. |
|
[6] Protocol for Studying Aqueous Foams stabilized by Surfactant Mixtures, J. Boos, W. Drenckhan, C. Stubenrauch, J. Surf. Det., 2013, 16, 1-12. |
|
[7] On how Surfactant Depletion during Foam Generation Influences Foam Properties, J. Boos, W. Drenckhan, C. Stubenrauch, Langmuir, 2012, 28, 9303-9310. |
|
[8] Foaming Properties of Mixtures of a Non-Ionic (C12DMPO) and an Ionic Surfactant (C12TAB), E. Carey, C. Stubenrauch, JCIS, 2010, 346, 414-423. |
|
[9] Mixtures of n-Dodecyl-β-D-maltoside and Hexaoxyethylene Dodecyl Ether: Surface Properties, Bulk Properties, Foam Films, and Foams (peer-reviewed Review), C. Stubenrauch, P.M. Claesson, M. Rutland, E. Manev, I. Johansson, J.S. Pedersen, D. Langevin, D. Blunk, C.D. Bain, Adv. Colloid Interface Sci., 2010, 155, 5-18. |
|
[10] Properties of Aqueous Foams stabilized by Dodecyltrimethylammonium Bromide (C12TAB), E. Carey, C. Stubenrauch, J. Colloid Interface Sci., 2009, 333, 619-627. |
|
[11] Aqueous Foams stabilized by n-Dodecyl-β-D-Maltoside, Hexaethyleneglycol Monododecyl Ether, and their 1:1 Mixture, C. Stubenrauch, L.K. Shrestha, D. Varade, I. Johansson, G. Olanya, K. Aramaki, P. Claesson, Soft Matter, 2009, 5, 3070-3080. |
Contact

Natalie Preisig
Dr.Scientific Staff

Cosima Stubenrauch
Prof. Dr.Dean of Faculty
- Profile page
- +49 711 685 64470
- Write e-mail
- Secretary: Room 9-302; Tel. +49 711 685-64451 and -64393; sekretariat2@ipc.uni-stuttgart.de