Overview
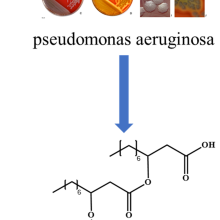
Due to their high solubilization capacities and the ability to achieve ultra-low interfacial tensions between water and oil, microemulsions are of great interest in fundamental chemistry and manifold applications, including washing processes, pharmaceutics and cosmetics. In contrast to emulsions (e.g. milk or butter), microemulsions are thermodynamically stable, optically transparent mixtures of an aqueous and an oily component, in which amphiphilic molecules such as surfactants are used for stabilization. Given the wide range of applications, large quantities of surfactants are released into soil, water and sediments, with many of them being petroleum-based. Several studies have shown that an increased content of synthetic surfactants in the environment has a strong impact on the ecosystem [5-7]. In this context, the class of natural surfactants is becoming increasingly important. Natural surfactants have a very high biodegradability due to their natural origin from bacteria, yeasts and fungi.
In comparison to a common linear alkyl benzene sulfonate surfactant , the critical micelle concentration of the biosurfactant rhamnolipid, is in a similar range around 10-1 mmol/L. Furthermore, the addition of the rhamnolipid lowers the minimum surface tension between water and air from 72 mN/m to about 30 mN/m for both surfactants. However, the properties of aqueous surfactant systems, emulsions and microemulsions stabilized by natural surfactants have generally only been studied to a limited extent.
The aim of this project is the formulation of efficient, sustainable and environmentally friendly microemulsions of the type H2O/salt – bio-oil – biosurfactant and to characterize their properties, starting from the application-relevant but not very sustainable microemulsion system, consisting of an aqueous CaCl2 solution, n-octane and a surfactant mixture of commonly used synthetic surfactants. As a first step, the hydrolysis stability of the used biosurfactants is investigated using various methods (e.g. 1H-NMR). Then, the influence of the type and size of hydrophilic head and hydrophobic residual groups of the biosurfactants on the solubilization efficiency are investigated. Since the biosurfactants are to be used in washing processes, their influence on the interfacial tension between water and bio-oils will also be systematically studied. In addition, the microemulsion nanostructure formed by the biosurfactant amphiphilic film will be elucidated using scattering methods such as light scattering and small-angle X-ray scattering, which is of particular interest in pharmaceutical drug delivery. Finally, existing phenomenological concepts such as the hydrophilic-lipophilic deviation (HLD) will be used to predict the influence of various parameters such as temperature and composition on the phase behavior, interfacial tension and structure of microemulsions.
References
Cooperations
- BASF SE, Ludwigshafen am Rhein, Germany
Contact

Florian Trummer
Doctoral Researcher

Abdulhamid Hejazi Almidani
Doctoral Researcher

Thomas Sottmann
Prof. Dr.Professor